By choosing TTMS, you are betting on experts who are well versed in quality management. We specialize in four key areas:
- validation of computerized systems,
- qualification of equipment and processes,
- ensuring the security of IT processes,
- and carrying out audits.
Our experience and advanced methods of work are a guarantee of high quality and safety at every stage of cooperation. We’re here to help your business achieve and maintain the highest standards.
TTMS Quality: Excellence in 4 areas
Evolve your processes and increase quality with TTMS’ comprehensive Quality approach.
We offer a comprehensive approach that includes four key elements:
Computerized Systems Validation
We provide strategic support and oversight as well as implementation model analysis for the design, implementation, maintenance and retirement of computerized systems in accordance with the restrictive requirements of Pharma GMP. We support software validation, ensure data integrity, manage software quality, and maintain validated status based on GAMP 5.0 and other guides.
Equipment & Process Qualification
We provide documented evidence of the compliance of the areas surveyed with the requirements. We confirm that equipment, installations, systems and rooms operate in accordance with the design and applicable GMP standards. We carry out qualification of process installations and media, laboratory installations, rooms, production and laboratory equipment, validation of the production process, analytical methods and qualification of cleaning.
Secure IT Processes
We support the management of organizations in the field of information security based on the ISO 27001 standard. We develop an implementation and operational approach for existing as well as for new areas, enabling the organization to be managed securely. We optimize processes in the organization while maintaining the required level of security.
Audits: We conduct audits of the organization or its systems for compliance with GMP, ISO 27001, 13785, 14007, 9001 and other standards. Our many years of experience allow us to assess requirements, implement solutions in organizations, prepare for accreditation audits and maintain compliance of processes with standards.
Audits
We conduct audits of the organization or its systems for compliance
with GMP, ISO 27001, 13785, 14007, 9001 and other standards. Our many
years of experience allow us to assess requirements, implement solutions in
organizations, prepare for accreditation audits and maintain compliance of
processes with standards.
Our areas of expertise:
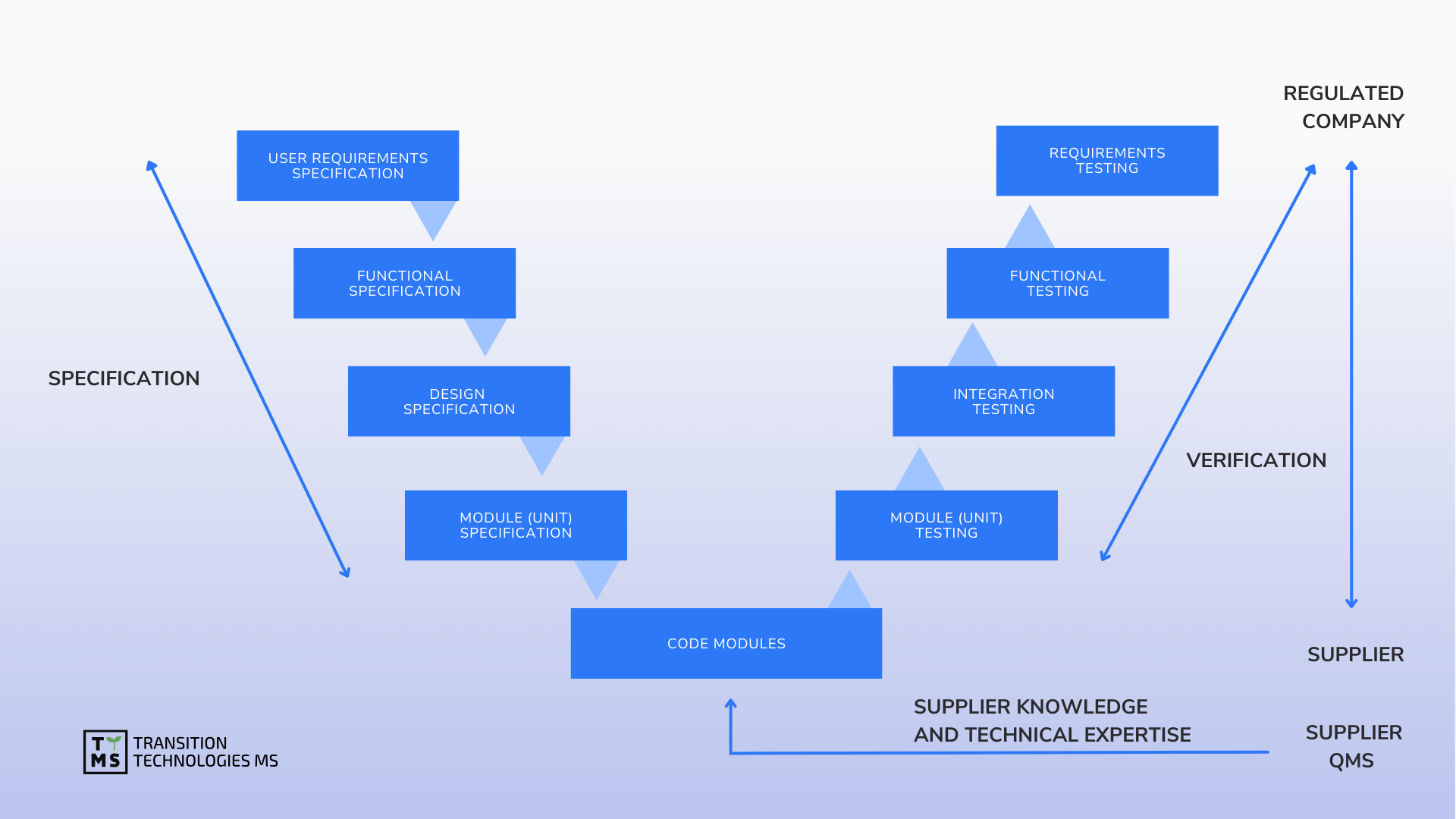
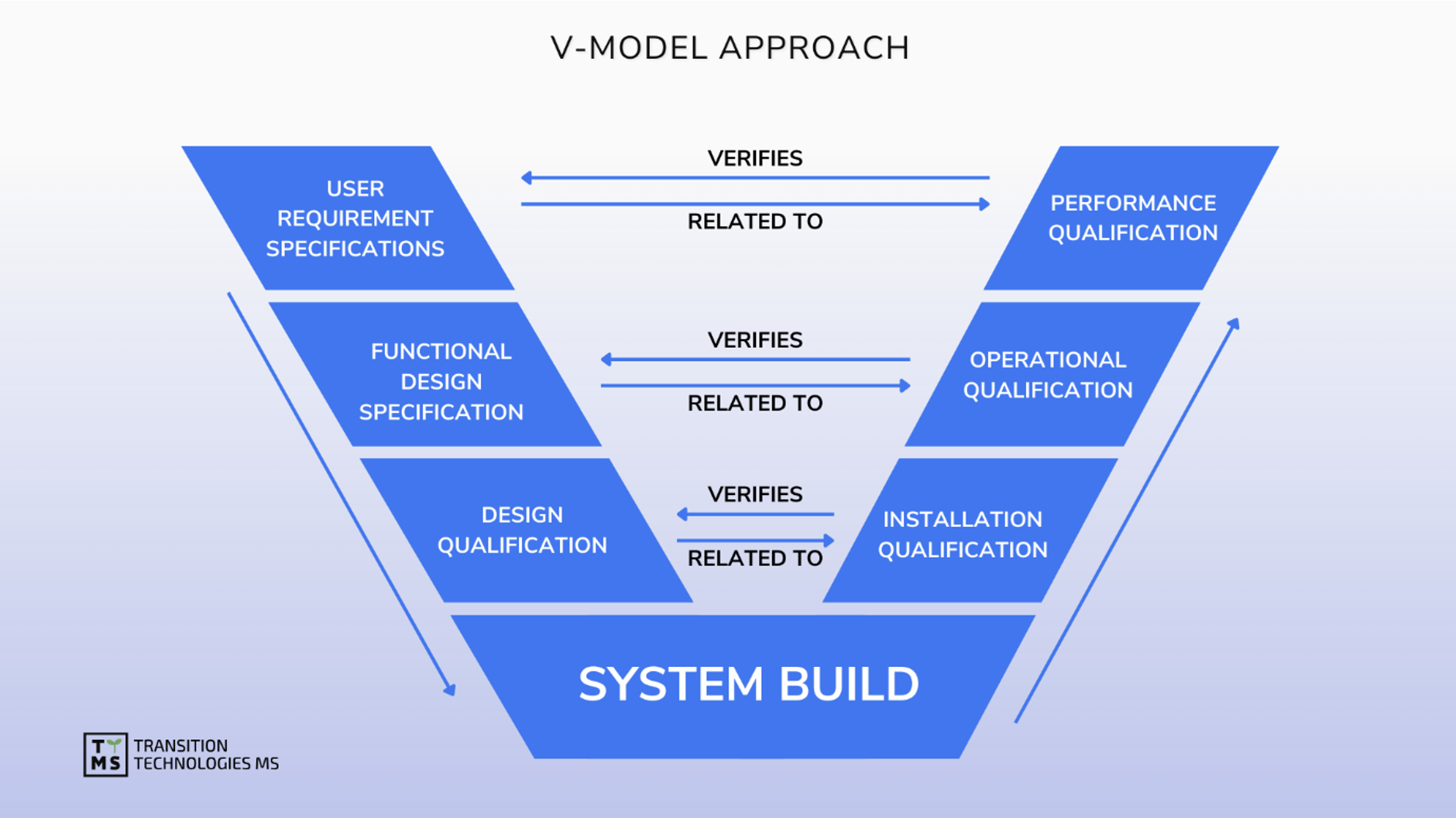
CSV Computerized System Validation:
Ready to take your business to the next level?
Let’s talk about how TTMS can help.

Sunshine Ang Sen Shuen
Sales Manager
